CONSERVATION OF NONFERROUS METALS
CONSERVATION OF NONFERROUS METALS
It is not uncommon to find nonferrous metals, copper, silver, lead, tin, gold, and their alloys, in archeological sites. These metals were used in the manufacture of art objects, coins, jewelry, and various utilitarian items such as fasteners, navigational instruments, cooking vessels, and small tools. They are more noble than iron and survive adverse environments in better condition than iron specimens. Perhaps it is for this reason that considerable attention has been given to their preservation and many conservation procedures have been developed for them. Nevertheless, the corrosion problems of each metal varies in different environments. Only those techniques applicable to the problems of sea-recovered metals are considered here.
As has already been observed, sea-recovered materials are often encapsulated by encrustation. However, when present on nonferrous metals, it is much thinner that what is found on iron. Of course, artifacts of these metals are often found encapsulated in the encrustation surrounding iron artifacts. Prior to any treatment of the metal artifacts, preliminary conservation steps must be completed. these include 1) initial documentation, 2) storage, 3) encrustation removal, and 4) artifact evaluation. The treatment of each metal group, i.e., cupreous metals, silver and its alloys, tin, lead and their alloys, and gold and its alloys, is discussed in some detail.
STORAGE OF NONFERROUS METALS
A variety of metal artifacts made of different metals are often found encrusted together in marine sites. In those instance it is necessary to store the material in such a way that the most susceptible metal is afforded protection, and little to no damage is done to the other metals and non-metals found in association. Since iron artifacts are the most commonly found metal, the storage conditions discussed under iron are most often utilized. However, gold, silver, pewter, brass, bronze, copper and lead artifacts; as well as ceramics, stone, glass, bone, cloth, seeds and wood are often all found together in various combination. In some cases, the best storage might be simply in fresh water. Once the different material are removed, then they are placed in the most appropriate storage environment for that material.
While iron artifacts, as discussed earlier, should at a minimum be stored in an alkaline solution shielded from light, this solution is not necessary or even recommended for artifacts made of other metals.
Copper is corroded by oxidizing solutions and in strong alkaline solutions. In neutral or slightly alkaline solutions the copper is passivated, the corrosion being checked by an oxide film that is formed on the surface. A 5% solution of sodium sesquicarbonate or sodium carbonate is recommended. A 5% solution of sodium carbonate with a pH of 11.5 will protect copper and silver. Silver is stable in aqueous solutions of any pH value and in the atmosphere, so long as these environments are free from oxidizing substances. Since chlorides do not affect lead or silver, once the encrustation has been removed, they do not need to be placed in an aqueous solution and can be stored dry. However, prior to the removal of adhering encrustation, it is best to house them in an appropriate solution to keep the encrustation from becoming harder and difficult to remove. It us quite safe to store silver objects in either a 5% sodium sesquicarbonate or sodium carbonate solution, along with iron artifacts. When silver is stored in a chromate solution, a film of brown Ag2O forms, which can be removed during the conservation, but for this reason, it is not recommended for the storage of singular silver artifacts. On occasion, it may be necessary for silver to be place in a chromate solution, when it is encrusted to an iron object.
Lead, tin and pewter are more easily stored. All are often stored dry, but as mentioned above, when the encrustation on metals is allowed to dry out, it becomes much harder to remove. For this reason, they are stored in aqueous solution. Lead is corroded by aqueous solutions free from passivating substances, especially soft water, deionized water, or distilled water. Thus, lead should never be stored in either deionized water, or distilled water, both of which are slightly acidic and lack any passivating substances in them. However since lead is corrosion resistant in hard, bicarbonate water because the bicarbonate is passivating, and both tin and pewter are passivated in slightly alkaline solutions, all can be stored in tap water with the pH adjusted to 8 - 10 by the addition of sodium sesquicarbonate. Both lead and pewter can be place in sodium carbonate, which has a ph of 11.5, but this pH is borderline to the corrosion domain of tin, so it should not be used for the storage of tin. Tin will resist corrosion in slightly alkaline solution free from oxidizing agents but will react adversely to strongly alkaline solutions. So any alkaline solution above 10 is potentially dangerous. Generally speaking tin can be safely stored in tap water. Lead, tin, and their alloys such as pewter should not be stored in a chromate solution because of its oxidizing effect which forms an orange chromate film on their surfaces, that is difficult to remove. In the absence of passivating substances, an oxidizing agent such as chromate can damage the specimen.
COPPER AND COPPER ALLOYS
CUPREOUS METAL CORROSION
The term cupreous metals is used to designate all the metals that consist of copper or copper alloys that contain copper as the predominant metal such as bronze (an alloy of copper and tin) and brass (an alloy of copper, zinc and often lead). The term does not imply a valence state as does cupric-divalent copper, or cuprous-monovalent copper. The cupreous metals are relatively noble metals that frequently survive adverse conditions, including long submersion in salt water, that often completely oxidize iron. They react with the environment to form similar alteration products such as cuprous chloride (CuCl), cupric chloride (CuCl2), cuprous oxide (Cu2O) and the aesthetically pleasing green and blue colored cupric carbonates, malachite [Cu2(OH)2CO3], and azurite [Cu3(OH)2(CO3)2] (Gettens 1964:550-557). In a marine environment, the two most commonly encountered copper corrosion products are cuprous chloride and cuprous sulfide. However, the mineral alterations in the copper alloys, bronze and brass, can be more complex than those of just copper.
The first step in the electrochemical corrosion of copper and copper alloys is the production of cuprous ions. These in turn combine with the chloride in the sea water to form cuprous chloride as a major component of the corrosion layer.
Cu? - e ? Cu+
Cu+ + Cl- ? CuCl
Cuprous chlorides are very unstable mineral compounds. Once cupreous objects are recovered and exposed to air, they inevitably continue to corrode chemically by a process commonly referred to as bronze disease. In this, cuprous chloride in the presence of moisture and oxygen are hydrolyzed to form hydrochloric acid and basic cupric chloride (Oddy and Hughes 1970:188).
4CuCl + 4H2O + O2 ? CuCl2 . 3Cu(OH)2 + 2HCl
The hydrochloric acid in turn attacks the uncorroded metal to form more cuprous chloride.
2Cu + 2HCl ? 2CuCl + H2?
The reactions continue until no metal remains. Any conservation of chloride-contaminated cupreous objects requires that the chemical action of the chlorides be prevented by removing the cuprous chlorides or converting them to harmless cuprous oxide. Otherwise, the artifact will self-destruct over time.
Copper objects in sea water are also converted to cuprous and cupric sulfide (Cu2S and CuS) by the action of sulfate reducing bacteria (Gettens (1964:555-556; North and MacLeod 1987:82). In anaerobic environments the copper sulfide products are usually in the lowest oxidation state as are the ferrous sulfides and silver sulfides. After recovery and exposure to oxygen, the cuprous sulfide to undergo subsequent oxidation to a higher oxidation state, i.e., cupric sulfide. The whole chemical reaction generally proceeds along the same lines as described for iron.
On removal from the marine encrustation copper and cupreous artifacts are inevitably covered with varying thicknesses of a black powdery layer of copper sulfide that imparts an unpleasing appearance. Occasionally, however, the corrosion process will create a pitted surface, but this is more common on the cupreous alloys where the tin or zinc is corroded preferentially, leaving the surface pits. The copper sulfide layer does not adversely affect the object after recovery from the sea like the copper chlorides do; they are primarily just disfiguring and may affect the shape and size of the object. The sulfide corrosion is easily removed and does not present the conservator with any major problems. See North and MacLeod (1987) for a detailed discussion of the corrosion of copper, bronze and brass in a marine environment.
CUPREOUS METALS
Here the non-specific term cupreous metals is used for copper and the alloys such as brass and bronze where copper predominates because of the difficulty of distinguishing copper, brass, and bronze objects from each other without analytical tests. In general it matters little what the specific alloy is, for they are usually treated in the same way. Care needs to be taken only when there is a high percentage of lead or tin, both of which are amphoteric metals and dissolve in alkaline solutions. There are a considerable number of chemical treatments for copper, bronze and brass, and most are not satisfactory for cupreous metals from marine sites. Consult the bibliography for further information.
In a marine environment the two most commonly encountered copper corrosion products are cuprous chloride and cuprous sulfide. However, the mineral alterations in the copper alloys are more complex than those of just copper. Once any cupreous object is recovered and exposed to the air, it continues to corrode by a process referred to as bronze disease. In bronze disease the cuprous chlorides in the metal in the presence of moisture and oxygen are very unstable. They hydrolyzed to form hydrochloric acid and basic cupric chloride. The hydrochloric acid in turn attacks the uncorroded metal to form more cuprous chloride. The reaction continues until no metal remains. Any conservation of chloride contaminated cupreous objects requires that 1) the cuprous chlorides be removed, 2) the cuprous chlorides be converted to harmless cuprous oxide, or 3) the chemical action of the chlorides be prevented.
Neither cuprous chloride or cuprous sulfide imparts a pleasing patina on the surface of the metals there is no reason to preserve them. In fact, most copper, bronze, and brass are darkly colored by sulfide, which often imparts a lead or pewter-like appearance to the metal. The stable copper sulfides only discolor the copper, imparting an unnatural appearance to the metal, and are easily removed with commercial cleaning solvents, formic acid, or citric acid.
In some instances, it is necessary to remove mechanically the gross encrustation and corrosion products down to the preserved surface of the metal. This step is facilitated for sea-recovered cupreous objects because the marine encrustation forms a cleavage line between the original surface and the encrustation. When the artifacts are removed from gross encrustation, superficial encrustation is often deliberately left adhering to the surface because of the artifacts' fragility or to avoid marring the surface. Careful mechanical cleaning and rinsing in water may be all that is required to remove this remaining residue. In other cases, all adhering encrustation is removed by soaking in 5 to 10% citric acid with 1-4% thiourea added as an inhibitor to prevent etching of the metal (Plenderleith and Torraca 1968:246; Pearson 1974:301; North 1987:233) Care must be taken as citric acid can dissolve cupric and cuprous compounds. The artifact is left completely submerged in the solution until the encrustation is removed. This may require an hour to several days, during which time the solution should be stirred to keep the acid concentration evenly distributed.
When a specimen is very thin, fragile, has fine detail, or is nearly or completely mineralized, any acid treatment may be too drastic. In these cases, the artifact can be soaked in a 5 to 15% solution of sodium hexametaphosphate (Plenderleith and Werner 1971:255) to convert the insoluble calcium and magnesium salts to soluble salts which can be washed away.
Following any necessary preliminary treatment, the conservation of chloride-contaminated cupreous objects requires that the adverse chemical action of the chloride be prevented. This can be accomplished by:
- removing the cuprous chloride
- converting the cuprous chloride to harmless cuprous oxide
-
sealing the cuprous chloride in the specimen from the atmosphere. The possible treatment alternatives include:
- galvanic cleaning
- electrolytic reduction cleaning
- alkaline dithionite
-
chemical cleaning
- sodium sesquicarbonate
- sodium carbonate
- benzotriazole
The first three techniques can remove cuprous chloride (CuCl) and reduce some of the corrosion products back to a metallic state; however, they are best used only on objects with a metallic core. If carefully applied, both will restore the object to a stable condition and maintain a form approximating its original uncorroded appearance. If misapplied they can strip the corrosion layer down to bare metal. Jedrzejewska (1963:135) draws attention to the fact that stripping, especially by electrolysis, may destroy significant archeological data such as tool marks, engraved lines, and decorative elements, as well as altering the original shape of the object. For these reasons the corrosion layers of any metal artifact should never be indiscriminately removed. The treatment should strive to preserve them in situ through very controlled electrolytic reduction or alkaline dithionite. The two chemical techniques described do not strip the corrosion layer. Rinsing in a sodium sesquicarbonate solution removes the chlorides, while benzotriazole and silver oxide seal the cuprous chlorides from the atmosphere. The chemical treatments are applicable to substantial objects as well as to completely mineralized pieces.
GALVANIC CLEANING
This procedure is carried out in exactly the same manner as described for iron. Since I regard it as an obsolete technique, except under certain circumstances already mentioned, there is no point to further discussion.
ELECTROLYTIC REDUCTION CLEANING
Electrolytic reduction of cupreous metals is also carried out in the same manner as described for iron. Either 2% sodium hydroxide or 5% sodium carbonate can be used for the electrolyte. The latter is used most often although acceptable results have been achieved using 5% formic acid as the electrolyte as described below for treating silver. A mild steel anode can be used but Type 316 stainless steel or platinized titanium is required if formic acid is used as the electrolyte. The same electrolytic setups described for iron or for silver are used.
The duration of electrolysis is shorter than for comparable chloride-contaminated iron objects. For example, small pieces such as coins require only a couple of hours while larger specimens such as cannons may require several months. Precise data concerning the current density are not available. Plenderleith and Werner (1971:198) state the current density should not be allowed to fall below .02 amps per square centimeter in order to prevent the deposition of a salmon-pink film of copper on specimens. Keel (1963:24) states that a current density above .01 amps per square centimeter will damage the specimens. Along these same lines Pearson (1974:301-302) correctly warns that care must be taken with mineralized bronze from under the sea when electrolytically cleaned in order to prevent damage to the surfaces by the evolution of hydrogen gas. Current densities within these given ranges and well in excess are commonly utilized on different objects. North (1987:238) recommends using the hydrogen evolution voltage techniques described for iron. In general the same procedure described for iron applies. The main difference being the much shorter time required to treat cupreous metals. Following electrolytic and chemical cleaning, cupreous metals are put through a series of hot rinses in deionized water. Because copper tarnishes in water, Pearson (1974:302) recommends washing in several baths of denatured ethanol. If a water rinse is used the rinsing can be followed by cleaning the tarnish with 5% formic acid or polishing with a paste of sodium bicarbonate.
After rinsing, the copper objects are dehydrated in acetone and are coated with a protective sealant such as clear acrylic. The commercially available Krylon Clear Acrylic Spray No. 1301 is recommended for ease of application, durability, and availability. Pearson's (1974:302) procedure of mixing 3% benzotriazole in the ethanol wash as an inhibitor against "bronze disease" followed by a clear acrylic lacquer containing the benzotriazole inhibitor (Incralac) is a good procedure to follow. The same protective sealant can be prepared by adding 3% benzotriazole to a solution of polyvinyl acetate (V15) in ethanol.
ALKALINE DITHIONITE
This treatment was devised for consolidating mineralized silver. Since then, it has been found to be effective on cupreous objects. See a complete description under Silver. The treatment destroys the patina, but it effectively removes the bulk of the total chlorides in the shortest period of time as well as reduce some of the copper corrosion products back to metal.
CHEMICAL TREATMENTS
Many cupreous specimens with chloride contamination, such as well-patinated bronzes with bronze disease, extensively mineralized bronzes with or without cuprous chloride, bronzes without a substantial metallic core, and bronzes with mineralized decorative features, cannot be treated by either of the reduction techniques. For these objects, three procedures are used to stabilize the artifacts while leaving the corrosion layers intact. They are: treatment with 1. sodium sesquicarbonate, 2. sodium carbonate, and 3. benzotriazole.
Sodium Sesquicarbonate
The cuprous chloride components of copper and its alloys are insoluble and cannot be removed by washing in water alone. When bronzes or other alloys of copper are placed in a 5% solution of sodium sesquicarbonate, the hydroxyl ions of the alkaline solution react chemically with the insoluble cuprous chloride to form cuprous oxide and neutralize any hydrochloric acid by-product formed by hydrolysis to produce soluble sodium chlorides (Organ 1963b:100; Oddy and Hughes 1970; Plenderleith and Werner 1971:252-253). The chlorides are removed each time the solution is changed. Successive rinses continue until the chlorides are removed. The object is then rinsed in several baths of deionized water until the pH of the last bath is neutral.
In practice, the superficial corrosion products are mechanically removed from the metal objects prior to putting objects in successive baths of 5% sodium sesquicarbonate, mixed with tap water in the initial baths, followed by deionized water in the subsequent baths. If the chloride contamination is extensive, tap water can be used until the Cl- increase in the solution approximates the Cl- level in the tap water. Then deionized water in substituted. This procedure is very economical when processing objects that require months of treatment.
In the beginning the baths are changed weekly; later the interval in extended. Monitoring the chloride level by the quantitative mercuric nitrate test, described under iron, enables the conservator to determine precisely how often to change the solution. In lieu of a quantitative chloride test, the qualitative silver nitrate test, previously described, can be used to determine when the solution is free of chlorides. The cleaning process is slow and may require months and in some cases even years.
Immersion in sesquicarbonate is followed by rinsing in several changes of distilled or deionized water until the pH of the last bath is neutral. The object is then dehydrated in acetone or a water-miscible alcohol, and coated with clear acrylic lacquer or microcrystalline wax. For increased corrosion protection, benzotriazole can be added to the drying alcohol and even added to lacquer.
The sodium sesquicarbonate method is often selected because, unlike other cleaning treatments, it does not remove the green patina of copper objects. Side effects, however, such as the formation of blue-green malachite deposits on the surface of the objects, can intensify the color of the patina. If this occurs, the object needs to be removed from the solution and the deposit brushed off. On some bronze pieces, there is considerable blackening of the surfaces, which obscures the original green patina and is difficult to remove. This blackening is attributed to the formation of black copper oxide and seems to be inherent in some cupreous alloys.
Sodium Carbonate Rinses
Sodium sesquicarbonate rinses, as described above, have been the standard treatment for fragile cupreous artifacts with chloride contamination and for artifacts that have a patina that is desirable to preserve. However, in practice, conservators found that it often enhanced the patina, making it much bluer in appearance. In other examples, it considerably darkened or blackened the patina. Recently, Weisser (1987:106) stated:
Although initially the sodium sesquicarbonate treatment seems to be ideal, since you do not need to remove the outer corrosion layers while the cuprous chloride is removed, it has been found to have a number of disadvantages. First, the treatment may require well over a year before all the cuprous chloride has been converted. This fact makes other drawbacks more serious. It has been shown that sodium sesquicarbonate (a double carbonate) forms a complex ion with copper and therefore preferentially removes copper from the remaining metal (Weisser 1975). This can be potentially structurally damaging over a prolonged period. It has also been shown that a mixture of carbonates, including chalconatronite, a blue-green hydrated sodium copper carbonate forms over the patina and also seems to replace other copper salts within the patina (Horie and Vint 1982) This creates a color change from malachite green to blue-green, which in many cases is undesirable. In the objects the author has examined the blue-green color can be found in cross section from the outer corrosion crust extending down to the metal substratum and Weiser (1987:108) concluded:
The stabilization of actively corroding archaeological bronzes remains a difficult problem for conservators. At the present time no known treatment can be called ideal. A sodium carbonate pre-treatment in conjunction with a standard treatment with benzotriazole offers one more option to the conservator who is faced with difficulties in stabilizing bronzes. Although successful stabilization has been achieved with this treatment where others have failed, it should be used with caution until the problems observed have been more thoroughly investigated. Bronzes which cannot be stabilized by this treatment should be stored or displayed in a low relative humidity environment. In fact it is recommended that all bronzes be kept in a low relative humidity environment if possible, since the long-term effectiveness of 'bronze disease' treatments has not been proven. Weiser suggests that if previous treatments with BTA have not been successful, then treat with 5% w/v of sodium carbonate in distilled water. The sodium carbonate removes the cuprous chlorides and neutralizes the hydrochloric acid in the pits. Sodium carbonate, unlike sodium sesquicarbonate, which is a double carbonate and acts as a complexing agent with copper, reacts relatively slowly with copper metal. Still, in come cases, slight alterations in the color of the patina can occur.
Benzotriazole
The use of benzotriazole (BTA) has become a standard part of any conservation treatment of a cupreous metal, following any stabilization process and preceding any final sealant. In some cases, it can be a single treatment unto itself, but when marine cupreous objects are conserved, it usually used as a final step in addition to some other treatment such as electrolytic reduction, or alkaline rinses which remove the bulk of the chlorides. In this method of cleaning (Madsen 1967; Plenderleith and Werner 1971:254) benzotriazole forms an insoluble, complex compound with cupric ions. The precipitation of this insoluble complex over the cuprous chloride forms a barrier against any moisture that could activate the cuprous chloride that causes bronze disease. The treatment does not remove the cuprous chloride from the artifact, it merely forms a barrier between the cuprous chloride and moisture of the atmosphere.
The process consists of immersing an object in 1-3% benzotriazole dissolved in ethanol or water. For artifacts from a freshwater site, it may be the only treatment required; it being used to prevent any future corrosion or discoloration of the patina. The BTA is usually dissolved in water, but ethanol can be used. See Green (1975), Hamilton (1976), Merk (1981), Sease (1978) and Walker (1979) for additional information. BTA forms an insoluble, complex compound with cupric ions. Precipitation of this insoluble complex over the cuprous chloride forms a barrier against any moisture that could activate the cuprous chlorides responsible for bronze disease. It has been found that if the artifact is left in BTA for at least 24 hours, 1% BTA mixed with D.I. water works as well as the stronger solutions. For shorter treatment, 3% BTA mixed in either water or ethanol in recommended. The main advantage of the ethanol, if there is one, is that it penetrates cracks and crevices better than water. In some cases ethanol is preferred when the BTA treatment is of short duration. In most situation the best results are achieved if the specimen is impregnated with the solution under a vacuum for 24 hours. On removing the object it is wiped off with a rag saturated in ethanol to remove excess benzotriazole. The artifact then is exposed to the air. If any fresh corrosion appears, the process is repeated until no adverse reaction occurs. Tests at the British Museum (Plenderleith and Werner 1971:254) indicate that if active bronze disease is present, all attempts to stabilize the object with benzotriazole may fail because of the widespread distribution of cuprous chloride CuCl in the corrosion layers. It has been found by any number or conservator that when cupreous artifacts from marine sites are treated, better long-term stability is achieved, if the bulk of chlorides are removed by either sodium sesquicarbonate rinses or sodium carbonate rinse and then BTA is applied and a final sealant, such as Krylon Clear Acrylic 1301 is applied. It has to be emphasized that the BTA treatment does not remove the cuprous chloride from the artifact, but merely forms a barrier between the cuprous chloride and the moisture in the atmosphere. Therefore, for artifacts heavily contaminated with chloride, such as marine recovered copper/brass/bronze objects, the treatment may have to be combined with one of the processes described above. Treatment by this method alone is not always successful but it is now a standard part of any treatment of copper or copper bearing alloys, in addition to any other treatment.. BTA is a suspected carcinogen and contact with the skin should be avoided and the powder should not be inhaled.
FINAL TREATMENT AND SEALANT
Following electrolytic or chemical cleaning the objects are put through a series of hot rinses in deionized water. Because copper tarnishes in water, Pearson (1974:302), recommends washing in several baths of denatured ethanol. If a water rinse is used any tarnish can be removed with 5% formic acid or by polishing with a wet paste of sodium bicarbonate (baking soda).
After rinsing, copper objects should polished to any degree desired, treated with BTA, dehydrated in acetone and sprayed with a protective coating of clear acrylic. Krylon Clear Acrylic Spray #1301, which is Acryloid B-66 in toluene, is recommended for ease of application, durability, and availability. For additional protection BTA can be mixed with Acryloid B-72 or polyvinyl acetate and brushed on the artifact. Microcrystalline wax can be used, but in most cases has no special advantage over acrylics.
SUMMARY
All the treatments discussed here are effective for the treatment of all artifacts from marine sites that containing copper. Each is effective in its own way and is the preferred treatment for given artifacts. Of the conservation alternatives considered in this section, electrolytic reduction, alkaline dithionite, and alkaline rinses are the only ones which actually remove the cuprous chlorides. For this reason they promise the most enduring protection. Electrolytic reduction cleaning of copper alloyed objects, brass and bronze, is often avoided because it removes any aesthetically pleasing patina and may change the color by plating copper from the reduced corrosion compounds on the surface of the alloyed metal. In some cases, as in the case of cupreous specimens from the sea, this is a small price to pay for a chemically stable artifact. My experience and the apparently successful application of electrolytic reduction to large number of copper and bronze artifacts clearly demonstrates that electrolysis is the quickest, the most effective, and the most enduring means of processing copper, brass or bronze objects from a salt water environment. This statement is especially true for larger objects such as cannons.
The extremely long time required for the sodium carbonate or sodium sesquicarbonate treatment discourages its use. A preliminary treatment of sodium carbonate, followed by treating with benzotriazole, may provide satisfactory results, but more experiments need to be reported before a final judgement can be made. Although preliminary, good result are being had with the use of alkaline dithionite solution treating cupreous alloys. It, like electrolytic reduction, has the ability to reduce copper corrosion products back to a metallic state and like the alkaline rinses, remove the soluble chlorides. This treatment may prove to be as useful for treating cuprous artifacts as silver artifact, for which it was originally developed. Regardless, of the mode of treatment, an application of BTA is an inherent part of the treatment of any cupreous metal artifacts. In most cases, if the artifact is effectively treated with any of the treatments discussed above, treated with BTA, sealed with an acrylic such as Krylon 1301 Clear Acrylic, and stored in the right environment, the artifact will remain stable.
SILVER CONSERVATION
SILVER CORROSION
Silver is a very noble metal and is often found in a native state combined with gold, tin, copper and platinum. It is completely stable in aqueous solutions of any pH as long as oxidizing agents or complexing substances are not present. Furthermore, it is not attacked appreciably by dry or moist air when the air is free from ozone, halogens, ammonia and sulfur compounds (Pourbaix 1966:393; Plenderleith and Werner 1971:239). Silver is particularly susceptible to the effects of the sulfide radical. This is most evidenced by tarnish on silver objects when exposed to sulfur in any form, but especially hydrogen sulfide and also sulfur dioxide which can convert to sulfuric acid. In a marine environment, with its abundance of soluble sulfates and oxygen-consuming, decaying organic matter, sulfate-reducing bacteria utilizes the available sulfate under anaerobic conditions to form hydrogen sulfides as a metabolic product. The hydrogen sulfide reacts with the silver to form silver sulfide. The overall reaction proceeds in the same process as described earlier for iron.
2Ag + H2S ? Ag2S + H2?
In anaerobic marine environments silver sulfide (Ag2S) is by far the most common mineral alteration compound of silver (North and MacLeod 1987:94). It is commonly reported from shipwrecks in the Caribbean and Australia and constitutes the most prevalent corrosion compound on the silver pieces from marine sites. In fact, a large percentage of the silver artifacts are completely converted to sulfide. Others have minimal metal remaining. Most have a thin sulfide surface layer which has removed some surface detail such as inscriptions, marks and stamps. In aerobic seawater the most commonly encountered corrosion products on silver and silver alloys are silver chloride (AgCl), and silver bromide (AgBr), with varying amounts of silver sulfide (Ag2S) (North and MacLeod 1987:94). Silver chloride is generally not extensive on silver recovered from salt water. Gettens (1964:563) notes that silver coins recovered from salt water are sometimes superficially altered to this mineral. I have observed only a few instances where silver chloride appeared to be present on some silver artifacts I have treated. In sites where the conditions vary between aerobic and anaerobic, combinations of all the major silver corrosion products are likely to be present (North and MacLeod 1987:94-95). In the case of relatively pure silver, silver sulfide (Ag2S) and silver chloride (AgCl) predominate. In the case of base silver alloys with significant amounts of copper, the copper corrodes preferentially, forming cuprous oxide, cupric carbonate, and cuprous chloride. In base silver alloys with copper, the copper corrodes preferentially and forms cuprous chloride which continues to corrode the copper component of the silver. In these cases the silver is treated as if it were copper. Regardless of what silver corrosion products are formed, all are stable and do not take part in any further corrosive reaction with the remaining silver. In fact, the corrosion layers impart some degree of protection from further corrosion to the metal. They also often provide an aesthetically pleasing patina which is often desirable and is deliberately preserved. The only reason to treat silver is to remove disfiguring corrosion layers to reveal detail, for aesthetic reasons, to reduce mineral products back to a metallic state, and to remove the chlorides from the copper component part of base silver alloys. Prior to conservation treatment, the marine encrustation is removed mechanically and in some cases by immersion in 10 to 30% formic acid solution. The conservation alternatives for cleaning silver and silver alloys are: 1) galvanic cleaning, 2) electrolytic reduction, 3) alkaline dithionite, 4) chemical cleaning, and 5) stabilization and consolidation.
GALVANIC CLEANING
Treating silver galvanically can be accomplished by using mossy zinc or aluminium in caustic soda as described earlier for iron. Variations include using mossy zinc or aluminium granules with heated 30% formic acid (Plenderleith and Torraca 1968:241-246; Plenderleith and Werner 1971:197 and 221). After treatment, the metal goes through an intensive rinsing, is dehydrated in a water-miscible solvent and is covered with clear acrylic lacquer. Galvanic cleaning is effective but has nothing to recommend it over electrolytic reduction or alkaline dithionite treatments.
ELECTROLYTIC REDUCTION CLEANING
The electrolytic cleaning of silver takes advantage of the reduction action of electrolysis by removing the chloride and sulfide ions from silver chloride and silver sulfide. When a direct current is applied, the negatively charged chloride and sulfide ions migrate toward the positive charged anode. The chlorides may form as chlorine in the solution and the sulfides oxidize to sulfates. Since the anions do not react with the inert anodes, they accumulate in the electrolyte and are discarded with it. During the process, the silver in the corrosion compounds is left in a metallic state.
Two methods of electrolytic reduction cleaning have been described in the conservation literature. Organ (1956) refers to the two as normal reduction and consolidative reduction. Normal electrolytic reduction uses a fully rectified direct current power supply. Consolidative reduction employs a partially rectified (asymmetrical) alternating current power supply. Both techniques require that a metal core be present in the object. Our laboratory has been concerned primarily with the normal reduction process in 5% formic acid, essentially as it is described in Plenderleith and Werner (1971:222). Both techniques are discussed below.
Electrolyte
Two electrolytes, formic acid (HCOOH) and sodium hydroxide (NaOH), are used to clean silver. Five to 30% HCOOH and 2 to 15% NaOH in deionized water have been proposed (Organ 1956:129; Plenderleith and Werner 1971:222; Pearson 1974:299). Either a 5% HCOOH or a 2% NaOH solution has been used as the electrolyte for cleaning silver.
Current Density
A current density of .01 amp per square centimeter, the same as proposed by Organ (1956:129), with good results. Plenderleith and Werner (1971:198) state that the current density should not be allowed to fall below .02 amps per square centimeter in order to prevent a film of salmon-pink copper from the corrosion crust, cathode screen, or copper leads from being deposited on the artifacts. Since the number and size of items being treated is variable, Pearson (1974:299) adjusts the current to produce a cell voltage of approximately three volts. In a series of experiments Organ (1956:134) found that a current density of 30 to 50 milliamperes/dm2 (.3 to .5 milliamperes/cm2) reduced more silver. However, North (1987:240) notes "...that the voltage applied during electrolysis does not appear to be critical, good results being obtained with a wide range of applied voltage." This just points out the obvious fact that silver is easily reduced in electrolysis, regardless of the voltage or setup. Still, in most cases a very low current density in the range proposed by Organ is best for maximum metal reduction.
Anode Material
When treating silver, inert anodes such as expanded platinized titanium, stainless steel No. 316, are preferable. In some of the older conservation literature carbon anodes are recommend, but these invariably break down in the electrolyte and cause problems, so they are no longer used. Platinized titanium is especially recommended for acid electrolytes because it is almost totally inert and does not react with the electrolyte. Its extremely high cost limits its widespread use, except on small specimens. It can be used in both alkaline and acid electrolytes. Stainless steel No. 316 is a good substitute as long as HCOOH is used as the electrolyte. If stainless steel is used in sodium hydroxide, it will oxidize after prolonged electrolysis, resulting in the destruction of the anode and the deposition of iron on the silver. If NaOH is used as the electrolyte, mild steel anodes are preferred to stainless steel. Mild steel anodes should not be used in formic acid as it will quickly break down and invariably result in iron deposition on the silver.
Cathode Contact
One often wants to avoid attaching a clip to small silver pieces in order not to scratch the surface. This is especially true for coins and delicate pieces of jewelry. Direct, individual connections can be eliminated by using a cathode conductor screen made of copper mesh (Figure 9. The specimen to be cleaned makes an electrical contact to the negative terminal through the cathode screen which is connected to the negative terminal. The areas of the screen not used for making contact with the specimen should be covered with silicone rubber. The rubber keeps the objects separated and cuts down on the exposed copper, thus reducing the problem of copper plating on the silver. The electrolytic cell can be set up in any of the alternatives described for iron. As for iron, the setup where artifacts are attached with clips to a cathode rod and sandwiched between two suspended anodes (Figure 5-D) is the most used. This electrolytic setup is useful for numerous pieces and can be used for coins and other small pieces.
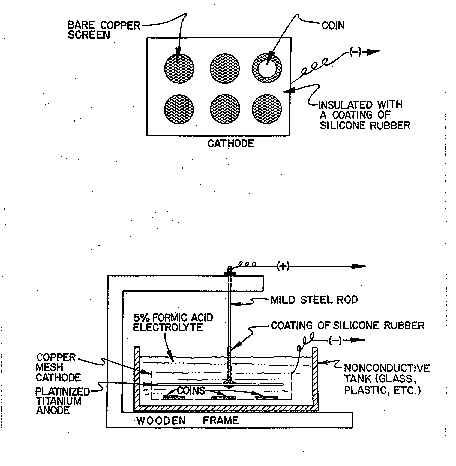
Figure 9. Electrolytic Setup for Cleaning Silver Coins or Other Small Artifacts.
|
Procedural Description
After the objects have been removed from the encrustation with a small pneumatic chisel, preferably using radiographs as a guide, they are thoroughly rinsed and are then ready to be cleaned electrolytically. Small specimens can be set up as shown in Figure 9. This setup is designed to clean coins, but is applicable to any small objects of silver or other nonferrous metals. The setup uses a glass container, a copper mesh cathode conductor screen, a wooden support frame for the anode, and an expanded platinized titanium anode attached to a mild steel rod. The rod is covered with silicone rubber to prevent its reacting, and insuring that only the inert platinized titanium anode acts as the anode. Alternatively, stainless steel No. 316 can be used as the anode. The specimens are placed on the cathode screen, the current applied, and the 5% formic acid electrolyte added. The current is never turned off while the coins are in the electrolyte in order to prevent any of the salts in the electrolyte from plating on the coin. This precaution will reduce considerably the problem of copper plating on the silver. Periodically the coins are removed, while the current is on, to be brushed under deionized water and dipped in a .2N solution of silver nitrate to remove any plated copper and superficial sulfide. They are then put back in electrolysis with the opposite side up. Electrolysis is continued until each side has a uniform appearance as determined by visual inspection, and hydrogen is fully evolving from the surface. Generally, small objects require only a few hours of electrolysis. Large silver objects or irregularly shaped pieces can be cleaned in the same way as described above, except that a cathode conductor screen is not used. The object is connected to the negative terminal with a clip. Due to cost factors, expanded stainless steel No. 316 mesh is more commonly used as the anode material than platinized titanium.
Reduction in Formic Acid
Organ (1956) conducted several detailed experiments on silver reduction techniques and alternatives. He notes that standard electrolytic reduction in 30% aqueous formic acid has been favored because it is a volatile acid that has no detrimental effect on silver and requires minimum washing after reduction. He found that when formic acid electrolyte is used at a current density of 1 amp/cm2 the reduced layer of transferred material external to the original surface delaminates, i.e., readily detaches along the original surface, revealing it. For this reason, treatment in a formic acid electrolyte is ideal for silver with the original surface preserved in the corrosion layer as long as a substantive metallic silver core remains. The reduced silver corrosion layers regenerated on the surface of the metal in formic acid are left in granular or particulated layers which are physically weak and tend to separate from the metal core. Clear acrylic lacquer is then applied to secure the layers in place on the surface to preserve the detail of the specimen. Being particulate, the reduced metal is dark, brittle, and not ductile, but the method of electrolysis in HCOOH results in a darkened silver that is stable, cleansed of corrosion products, and yet still looks old. If a brighter surface is desired, the silver can be lightly polished with a paste of sodium bicarbonate, a fine fiberglass brush, or a silver buffing cloth.
Reduction in Sodium Hydroxide
Reduction in a 3 to 15% aqueous solution of sodium hydroxide at a low current density (10 to 50 milliamps/dm2) is said (Organ 1956:135) to result in firm, hard, metallic silver capable of being polished. The regenerated silver retains the detail and texture of the original laminated corrosion surface but it is full of voids and is not ductile. More recent tests have shown that more thorough reduction is achieved in a NaOH electrolyte.
Consolidative Reduction
Fully rectified direct currents have been used in conservation, electroplating and battery charging, but it was discovered some years ago that a small amount of reverse current (also called partially rectified or asymmetrical alternating current) produces smoother electroplated finishes, faster battery charging time, and increased battery life. The technique was first described in the conservation literature by Organ (1956) as consolidative reduction.
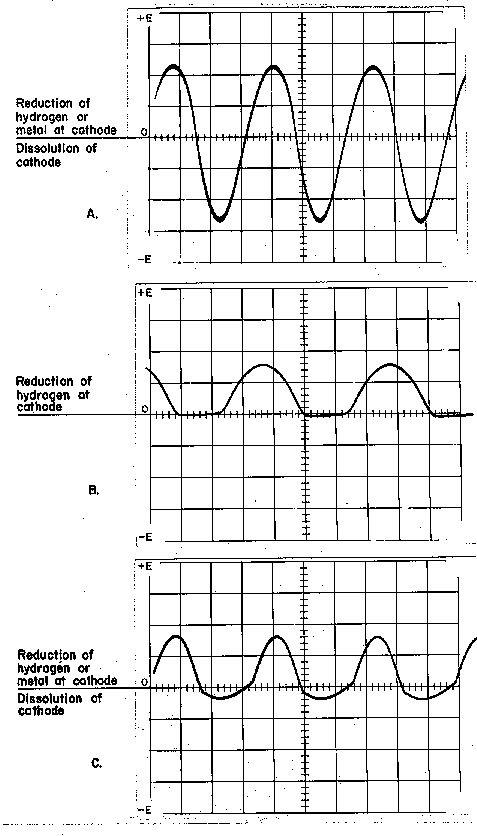
Figure 10. Electrical Sine Wave Forms.
|
reverse current (current flow from positive to negative); therefore, it has a symmetrical sine wave form. If an artifact is undergoing electrolysis, hooked up to alternating current, metal and hydrogen are deposited and metal is reduced from the corrosion compounds during the forward half of the cycle. In the subsequent reverse half of the cycle the metal and hydrogen deposited or reduced are dissolved. No progress in reduction takes place.
Direct current flows only in a forward direction, therefore only reduction and deposition reactions take place at the cathode (Figure 10-B). In normal reduction using direct current, metal and hydrogen are reduced at the surface of the specimen being treated, but in the process the cathode can become polarized by the accumulation of hydrogen gas bubbles formed and deposited at the cathode surface. The hydrogen gas can insulate the surface in some areas, while other areas are in direct contact with the electrolyte. This provides for uneven metal deposition and microscopic voids in the newly reduced metal. In consolidative reduction 10-20% reverse current and 80-90% forward current is usually used. During electrolysis the net effect is a rapid succession of reduction and dissolution cycles (Figure 10-C). During the 90% forward half of the cycle, reduction of metal in the corrosion compound and deposition of metal dissolved in the previous reverse current half cycle takes place. During the 10% reverse half cycle there is a partial dissolution of the previously reduced or deposited metal; however, the 90% forward current puts the emphasis on reduction and deposition over dissolution as the current reverses 120 times a second. In the process the extent of polarization of the cathode is reduced. Organ (1956) used asymmetrical alternating current in a sodium hydroxide electrolyte to regenerate the silver on the Ur lyre that was completely mineralized to silver chloride to massive metallic silver while preserving the surface details of the corrosion layers. The reduced silver was ductile and more homogeneous than the silver reduced by normal reduction techniques using fully rectified direct current. He used a 3% NaOH electrolyte, a carbon rod anode, and a very low current density of 10 milliamps/dm2 (.1 ma/cm2) to reduce the silver and to prevent the rapid evolution of hydrogen that would tend to disturb the reduced silver.
For badly or completely corroded specimens, more complete reduction is achieved if the cathode wire is laid against one side of the silver and the exposed wire covered with wax or polymethacrylate. This insures that the current passes through the corroded metal in flowing from the electrolyte to the cathode. The hydrogen discharges at the surface of the mineralized metal and reduces it. Organ (1956) resorted to this technique in order to make an electrical contact with the non-metallic, poor-conducting silver chloride on completely mineralized silver. The arrangement is advantageous even when a thin core of metallic silver remains. During the process, the corrosion layers external to the original surface are reduced in place, preserving all the details present on this surface. The treatment usually requires weeks. Since this technique preserves all the outer corrosion surface, it should not be used on specimens with an original surface preserved within the corrosion crust. Following reduction the piece is rinsed in cold deionized water to remove all the alkali and then coated with any suitable sealant.
Additional details concerning the development and application of consolidative reduction are to be found in Organ (1956:137-144), in summary form in Plenderleith and Werner (1971:223-226), and additional research presented in Charalambous and Oddy (1975). The description of the circuit for the partially rectified current is provided in both sources. Asymmetrical alternating current seems to have some advantages over straight direct current, and may prove to be superior when used to treat any metal artifact, even iron objects. More experimentation is needed, but in general, even though this option of using asymmetrical current seems to have some advantages, it has never been adopted very widely by conservation laboratories since reduction of silver corrosion products back to metallic silver can be achieved with very low current densities using straight D.C. current and a NaOH electrolyte.
ALKALINE DITHIONITE
The alkaline dithionite treatment is similar to that of alkaline sulfite described for iron. Is a relatively cheap, simple and rapid method of consistently reducing silver corrosion product to metallic silver (MacLeod and North 1979). The steps in the processing of silver by this method are:
- Immerse the object in 10-12% hydrochloric acid to remove the encrustation layer that may consist of sand, shell, calcium carbonate, and copper and iron corrosion compounds. This requires from 12 hours to a week or until all cleaning action ceases and no more gas bubbles evolve. During this step it is necessary to make sure that the solution remains acidic. If necessary, concentrated hydrochloric acid is added to the solution to maintain a working strength.
- Rinse thoroughly in tap water to remove all residual encrustation and mechanically remove any stubborn spots.
- Immerse in a solution of alkaline dithionite. Mix up a solution of sodium hydroxide (40 g. sodium hydroxide per liter of water). Once the sodium hydroxide dissolves add 59 g. of sodium hydrosulfite and then immerse the silver quickly to eliminate oxidation of the solution in the container. The container should be completely full of solution and have an air tight seal. The amount of sodium hydrosulfite is not critical -- so any thing in the 55-65 gram range will be effective.
- For one week agitate and turn the container daily to keep the solution mixed and to expose all surfaces of the specimens to the solution.
- After one week remove and rinse the specimens in water until the pH of the rinse water remains unchanged.
- The corrosion products on the surface of the artifact will be reduced to a grey, metallic silver which can be polished with a wet baking soda paste or a fiberglass brush.
In addition to being very effective for reducing silver corrosion products, the alkaline dithionite effectively reduce copper corrosion compounds on badly corroded copper and bronze objects and reform the original surfaces. It has been used successfully on all cupreous artifacts, converting copper corrosion products back to metallic copper.
To dispose of the used solution, allow it to air-oxidize for several days to oxidize the sulfites to sulfates. Then neutralize it with the left-over Hcl. It can then be safely disposed in the drain; however, by using electrolyitc reduction, it is possible to extract all the silver from the solution -- by plating it on a cathode. The money received from the sell of the silver and come close to paying for the treatment.
RINSE AND SEALANT
Following electrolysis, the specimen is rinsed with deionized water. If an alkaline electrolyte is used the rinsing should be quite intensive, otherwise, a white precipitate can form. The silver is dried with hot air or dehydrated in acetone and then coated with clear acrylic lacquer such as Krylon 1301.
CHEMICAL CLEANING
The majority of silver objects recovered from archeological contexts require only limited treatment. In most instances the various corrosion products can be removed with simple chemical solutions (Plenderleith and Werner 1971:227-229). Common tarnish caused by sulfur compounds can be eliminated easily with commercial silver cleaning solutions. Alternatively, a mild silver dip solution can be prepared that consists of 5% thiourea and 1% non-ionic detergent in distilled water. A solution of 15% ammonium thiosulfate in distilled water with 1% non-ionic wetting agent is stronger than the silver dip and is effective for removing both tarnish and silver chloride. For base silver with copper corrosion compounds, concentrated ammonia effectively cleans all copper compounds from the silver. Care must be taken, however, because it dissolves silver chloride and will substantially weaken badly corroded silver. Formic acid in a 5 to 30% aqueous (deionized water) solution is effective for dissolving copper compounds without affecting silver chloride. Formic acid also can be used to brighten silver that has been cleaned with some other chemical or other technique. Silver nitrate solution is used for removing films of metallic copper. Often just simple washing in soapy water or rubbing with a mild polishing abrasive is sufficient.
STABILIZATION AND CONSOLIDATION
Since silver sulfide and silver chloride are stable compounds, corroded silver pieces do not need to be stabilized. Consolidation, however, is often required. Many of the silver coins and other small silver pieces likely to be found within an encrustation have completely converted to silver sulfide. In some cases all that remains of the silver is a wet slush without form or structural integrity. In a few cases an enlarged, deformed, or discontinuous crystalline structure remains, and all that can be done is to record any data contained as an impression of the coin in the surrounding encrustation. In many instances these data can be preserved by making a case of the impression.
Where the object is nearly or completely converted to a compact, cohesive silver sulfide, the form and all the details and marks of the original specimen are retained. All that may remain of some coins is a light silver sulfide wafer that can be crumbled to powder with slight pressure. If consolidative reduction is not attempted, or is impossible, any cleaning treatment would destroy the coin or at least destroy all the markings and details preserved only in the mineralized sulfide layer. In some instance it may be possible to conserve the in the alkaline dithionite solution described above. In other instance, the only alternative is to consolidate the sulfide. This is easily accomplished by placing the sulfide coin in acetone to dehydrate it. The coin is then placed in a dilute solution of polyvinyl acetate (PVA) and acetone. It is left in the solution until bubbles cease to rise; then the coin is removed, allowed to partially dry and the process in then repeated two or three times. The repeated immersions and drying assures that a maximum amount of the acetate is absorbed. Upon drying thoroughly, the treatment is completed. The PVA consolidates the sulfide layers, although the coin remains fragile and can be easily broken. Any number of other consolidants such at butyl acetate, various polymethacrylates, or even wax, can be used.
SUMMARY
Since the corrosion products of silver are stable, the treatment accorded silver artifacts is less critical. In some instance, however, when treating base silver with a significant amount of copper, it is the copper and its corrosion products that create the problem, and in these case, the artifact should be treated as copper. In many instance silver may be treated exclusively by mechanical means or by various chemical treatments. Because of silver's susceptibility to corrosion in anaerobic environments so characteristic of marine environments, a treatment is often employed that will reduce the silver corrosion products back to a metallic state. If reduction is the objective, then only electrolytic reduction and the alkaline dithionite treatments are applicable, and for this reason, they are the treatments most often used to conserve silver artifacts recovered from a marine environment. Each is effective in its own way, and the decision to use either one is base on the particular set up of the laboratory, and the number of artifacts to be treated.
LEAD, TIN, AND LEAD ALLOYS
TIN, LEAD AND LEAD ALLOY CORROSION
Articles of tin are seldom encountered in archeological sites. This metal is found more often used in various alloys, especially in combination with copper for bronze and with copper and/or tin for pewter.. Gettens (1964:560) notes that tin seldom survives because of the transformation of tin by direct intercrystalline oxidation to mixed stannous and stannic oxide (SnO and SnO2) or by allotropic modification to a loose powdery gray tin, commonly referred to as "tin pest." The alteration compounds of tin in a marine environment have not been adequately studied; it is known, however, that sodium chloride also stimulates the corrosion of tin. Off the coast of Turkey George Bass (1961) found probable ingots of tin completely oxidized to tin oxide. Although not often mentioned in literature, stannous sulfide can be expected to be found where sulfate-reducing bacteria are active in anaerobic environments.
Lead is commonly found in shipwrecks where it was used for weights, cannonballs, sheeting, and stripping. Lead is a stable metal in neutral or alkaline solution free from oxidizing agents especially if carbonates are present in the water (Pourbaix 1966:488-489). During prolonged exposure under most archeological conditions where there is exposure to the atmosphere, basic lead carbonate (2PbCO3 . Pb(OH)2) and lead oxides (PbO & PbO2) are formed. The gray lead carbonate and lead oxide generally form a protective layer that prevents further oxidation. Both these corrosion compounds are found on lead from a marine environment, but lead chloride (PbCl2) and especially lead sulfide (PbS) and lead sulfate (PbSO4) are common.
Gettens (1964:558) noted that few occurrences of lead sulfide have been reported, but more recent research (North & MacLeod 1987:89) report that the main lead corrosion products in anaerobic marine environments is lead sulfide, while in aerobic seawater it is lead sulfate, as would be expected. In marine environments, it is not unusual to find the remains of lead straps that have been completely converted to a black slush. The bulk of the corrosion is probably lead sulfide which results from the action of sulphate-reducing bacteria, as explained for iron. Possibly some intermediate forms of the lead oxides (PbO & PbO2) are formed, and oxysulfides are present. Lead often exhibits extensive corrosion attack when it is in contact with wood. For instance, I have seen lead strips that were nailed onto a ship's keel that were very badly deteriorated. Evidently the oxygen-consuming, decaying wood and the marine encrustation that forms over the lead created the anaerobic conditions conducive for the metabolism of the sulfate-reducing bacteria and the decaying wood provides nourishment for the bacteria. Most of the lead corrosion products, except white lead (Pb3(CO3)2(OH)2), which has not been found on lead artifacts form marine sites, do not adversely affect the artifact after recovery. They may be unsightly or even disfiguring, but they do not take part in chemical reactions that attack the remaining metal. The objects need to be cleaned for aesthetic reasons and possibly to reveal surface details under or in the corrosion layers. The corrosion products themselves are stable.
Lead alloys such as old pewter, which was formerly an alloy of tin and lead, oxidizes to the same compounds as the two parent metals. In archeological sites the condition of different pewter pieces varies widely, primarily because of different local conditions and varying percentages of tin to lead. In marine environments leaded pewter always survives in better shape than does lead free pewter, probably because of the formations of a protective lead sulfate (PbSO4) Lead free pewter suffers extensive corrosive attack in aerobic seawater; in fact pewter is commonly completely mineralized as stannic oxide (SnO2), lead sulfide (PbS) and various very brittle, mineralized antimony, tin (SbSn) compounds are formed. In contrast, in anaerobic environments, both leaded and lead-free pewter survives in good condition through the protective formation of lead and tin sulfide films (North and MacLeod 1989:90-91). In fact the only corrosion may be a thin sulfide film on the surface of well preserved metal. Various combinations of lead carbonate, lead oxide, lead sulfide, lead chloride, and tin oxide are possible. Pewter objects often have wart-like blisters on the surface of the metal which possibly result from localized contaminations of salts (Plenderleith and Werner 1971:278). These should not be removed, for under most of them there are either holes or pits in the metal.
CONSERVATION OF LEAD, TIN AND PEWTER
Once recovered from the sea, the corrosion products of objects of lead, tin and their alloy, pewter, are stable. The corrosion products may be unsightly or even disfiguring, but they do not take part in chemical reactions that attack the remaining metal. The objects need to be cleaned only for aesthetic reasons and to reveal surface details under the corrosion layers. Old pewter, being an alloy of lead and tin needs to be treated as tin, which is the more anodic and chemically sensitive metal. Therefore, no acids, or sodium hydroxide should be use, unless, in the case of electrolysis, the metal is given cathodic protection.
CHEMICAL TREATMENT OF LEAD
Because of the ease of treatment and the availability of the chemicals, the most widely used treatment for lead from any archaeological environment is the acid treatment described by Caley (1955). The same treatment can be used on tin or pewter. The lead is immersed in 10% hydrochloric acid which removes the adhering marine encrustation, along with lead carbonates, lead monoxide, lead sulfide, calcium carbonate, and ferric oxide. If lead dioxide is present, it is removed by soaking the object in 10% ammonium acetate. The ammonium acetate also acts as a buffer to protect the lead from the action of any hydrochloric acid that may remain. Lead, if placed in ammonium acetate, should be left in the solution only as long as necessary, as the solution can etch the metal. For most lead objects, the ammonium acetate step is not required. This treatment is good for lightly corroded specimens and it gives lead surfaces a pleasing appearance. The surface detail that is preserved by this treatment varies with the degree of corrosion when recovered. For more diagnostic lead artifacts, Caley's method has been superseded by electrolytic reduction, which has the ability to convert mineral products back to a metallic state. However, for the general cleaning of lead, without a lot of hands-on labor, it remains a much used and acceptable technique provided that all residue of the HCL is removed by rinsing in tap water, so that there is no contamination of any chloride sensitive material that it may be stored with. After using the HCL treatment on lead, one can still has the option to use electrolytic reduction to reduce any corrosion layer that are still in place back to a metallic state. In the objective is to completely remove all the lead corrosion products from a lead object, then a 5% solution of ethylenediaminetetraacacetic acid (EDTA) disodium salt is most effective. After complete immersion in the EDTA solution for 2-3 hours, or in some cases up to 24 hours for larger pieces, the object is then rinsed in tap water.
GALVANIC CLEANING OF LEAD
Any solid object of tin can be cleaned galvanically or by electrolytic reduction in the same way as described for iron and the other metals. Normally, in galvanic cleaning, the vat with the electrolyte, anodic metal, and specimen is heated to speed the reaction, but since tin is an allotropic metal that is slightly soluble in NaOH, heating should be avoided and the duration limited. Tin coins respond well to cold electrochemical reduction, using zinc, aluminium, or magnesium powder in caustic soda (Plenderleith and Werner 1971:275). Magnesium is often substituted for zinc since zinc sometimes discolors the tin (Plenderleith and Organ 1953). However, if electrolytic reduction equipment is available, there is little reason to consider using galvanic cleaning for any object of lead, tin, or their alloys.
In general, galvanic cleaning does not play much of a role in a properly equipped conservation laboratory.
In cases of badly oxidized tin objects, the only alternative is to consolidate in microcrystalline wax, or embed in a plastic material. Slow, extended diffusion of chlorides in alkaline solution are not a consideration because of the solvent action of the solutions.
ELECTROLYTIC REDUCTION CLEANING
The ability to control the reaction speed through current controls in electrolytic reduction makes it especially useful for lead coins and medals - any specimen where surface detail is important or reduction and/or consolidation of the corrosive layers is the objective. Two electrolytic reduction techniques, normal reduction (Plenderleith and Werner 1971:267-268) and consolidative reduction (Organ 1963a:131; Plenderleith and Werner 1971:268-270), are used for treating lead.
Normal Reduction
Lead artifacts with substantial metal can be cleaned by the normal reduction process using 5% sodium hydroxide, anodes of mild steel or stainless steel, and a current density of 2 to 5 amps/dm2. Very satisfactory results are achieved by this technique, but since lead is susceptible to solvent action by the electrolyte when it is not cathodically protected the current must be flowing before putting the specimen in the electrolytic tank and must not be cut off while it is immersed in the tank. A good electrical contact, as indicated by evolution of hydrogen from the object, must be made with the lead and it should be supported well enough to insure that the electrical contact is maintained. An initial high current density will remove the lead corrosion layers; therefore, when a low current density should be used to consolidate the corrosion layers through reduction. Since lead, tin and pewter are susceptible to attack by strong alkalies, a sodium carbonate electrolyte is safer to use. For instance, if the electricity were to go off and the lead or tin object, or alloy were in NaOH, it would be attacked. If sodium carbonate was being used as the electrolyte, a passivating film of carbonate would form and the attack would stop. The attack on tin and tin alloys by sodium hydroxide solution is particularly aggressive. Since sodium carbonate does a reasonably good job on artifacts made of these metals, the use of sodium hydroxide electrolytes should be reserved for consolidative reduction on those special artifacts where there is some reason to try to achieve that absolute maximum amount of corrosion products reduced back to metal. For example where there are inscriptions or marks that are preserved in the corrosion layer, then, sodium hydroxide should be used as the electrolyte. For the cleaning of well preserved lead, sodium carbonate is effective. This applies to either normal reduction or consolidative reduction.
Consolidative Reduction
This technique was developed by Organ (1963a:131) to consolidate the inscriptions contained in a fragile corrosion layer of basic lead carbonate on a group of lead seals. The removal of the corrosion layer would have obliterated the inscription. Consolidative reduction converts the basic lead carbonate and other lead corrosion products to a compact mass of lead. The object is tightly compressed between two polyurethane foam pads in order to support and put pressure on the corrosion layers while they are cathodically reduced at a current density of 100 to 200 milliamps/dm2. In the process, 5% sodium hydroxide electrolyte with stainless steel anodes is recommended. In consolidative reduction, which utilizes very low current densities, mild steel anodes can not be used because the current flow is so low that there is no way to keep the anodes passivated and they quickly disintegrate through a process called anodic dissolution. The procedure described by Plenderleith and Werner (1971:268-269) using a 10% solution of sulfuric acid with a lead anode is not used that often, because of the difficulties of handling sulfuric acid and the deposition of lead from the anodes on the artifacts being treated. In addition, more recent research has shown that there is more thorough reduction when NaOH is used as the electrolyte. Plenderleith and Werner (1971:269) suggest using a partially rectified alternating current source, which provides a "bumping" effect, for better results, but as discussed under silver, the use of asymmetrical A.C. current is not widely used since low current density electrolysis using straight D.C. current effectively reduces lead corrosion products back to metallic lead, especially when sodium hydroxide is used as the electrolyte. The use of asymmetrical A.C. current does not seem to increase the degree of reduction (Lane 1975; 1979). The important thing to remember is make sure that a cathodic protection is maintained by a flow of electrons to the lead or tin metal is being treated at all time.
Rinsing Procedure Following Electrolytic Reduction
The NaOH residues of the electrolyte cannot be removed completely from lead by rinsing in water alone; and a more complex procedure must by followed (Plenderleith and Werner 1971:269-270). The object is submerged in a dilute solution of sulfuric acid (15 drops of concentrated H2SO4 per liter of tap water) with a pH of 3 to 3.5 which neutralized the alkalinity of the electrolyte and also forms a protective coast of lead sulfate on the surface of lead objects. The artifact is taken through a succession of H2SO4 baths until the pH ceases to rise from the alkali diffusing from the lead. After the removal and neutralization of the alkali the lead is left acidic. The residual acidity is removed by immersion in successive baths of cold distilled water with a pH of about 6, until the pH of the water does not drop.
SEALANT
Following the rinsing, the reduced object is dried with hot air or dehydrated in a water-miscible solvent. The fragile reduced lead is then strengthened as well as protected from atmospheric corrosion by submersion in molten microcrystalline wax.
STORAGE
Lead is particularly susceptible to organic acids, such as acetic acid, humic acid, and tannic acid. Lead artifacts, therefore, should not be stored in oak cabinets or drawers. If so, even small concentrations of vapors of these acids can initiate corrosion, which proceeds rapidly. To be safe, lead should by stored in sealed containers or polyethylene bags.
Old pewter, being an alloy of tin and lead, can be treated by any of the methods described for lead. Electrolytic reduction is particularly recommended in order to reveal or preserve surface details, maker's marks, and designs. For well-preserved lead, cleaning with HCL or EDTA are effective. Of the metals recovered from marine sites, they preserve the best and are one of the easier to treat, in most circumstances.
Like silver, because the corrosion products of lead artifacts recovered from marine environments are stable, the exact mode of treatment is less critical. For this reason, treatment with hydrochloric acid (Caley's Method) is often employed because of its simplicity and effectiveness. For the same reason EDTA, disodium salt, is also effective for clean lead, as well as reasonably well preserved tin and pewter. However, still electrolytic reduction is the treatment most often employed because of the possibility of reducing some of the lead, tin, pewter corrosion products back to a metallic state. The exact mode of treatment depends on the condition of the artifact, the laboratory and the decision of the conservator. Each of the treatments are effective and are selected with some regularity.
GOLD AND GOLD ALLOY CONSERVATION
GOLD CORROSION
Gold, being a relatively inert metal, undergoes minimum corrosion. It is the copper and/or silver-base gold alloys that easily corrode, resulting in the same silver or copper corrosion compounds leaving an enriched and possibly weakened gold surface.
GOLD CONSERVATION
Pure gold and high gold alloys do not require any treatment. All the gold objects that I have seen from shipwreck sites appear to look the same when recovered as the day they went down with the ship. The copper and silver in low alloy gold do corrode. When present, the copper and/or silver corrosion compounds of low alloy gold are treated by the processes described for these two metals. Silver corrosion products can be removed with ammonia; copper compounds with formic acid, citric acid, or alkaline sequestering agents such as Rochelle salts or alkaline glycerol. All the pertinent comments applicable about silver and copper conservation are made under those headings.
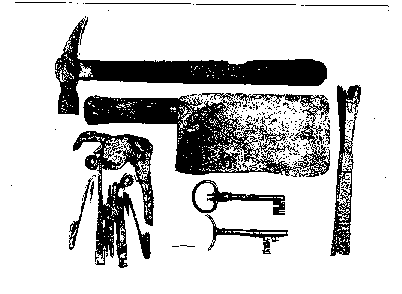
Figure 11. Epoxy casts of iron tools from the submerged town of Port Royal, Jamaica. From top to bottom and left to right: a hammer with the original wood handle, a cleaver with the original wood handle. a door lock, two keys, and a socketed chisel.
|
CASTING AND MOLDING IN CONSERVATION
The techniques of casting and molding are often used to restore and replicate specimens. Casting replicas for exhibition, distribution, and study is only an adjunct to conservation. This aspect of casting, although of considerable importance, is not considered here and the reader is referred to publications and brochures that can be obtained from manufacturers of casting materials, and to articles by Rohner (1964, 1970), Frazier (1974), Rigby and Clark (1965), and Hamilton (1976).In the conservation of marine archeological specimens, casting is resorted to when the artifact itself cannot be treated. In some cases, only through casting can the object be saved or its form determined. As explained earlier, metal objects within an encrustation can continue to corrode until little or no metal remains. In such cases, the original surfaces with identification marks, stamps, letters, or numbers are lost. Fortunately, the encasing encrustation begins to form immediately at the onset of the corrosion process, forming a mold and preserving details of the original form as well as marks or stamps on the surface.. Quite often, the encrustation is more informative than the deteriorated or badly oxidized object. Several ways of retrieving artifactual data are discussed. A number of different casting materials from many different manufacturers can be used. Through the use of many, we have come to rely on just a few. The materials include Dow silicone rubber, Smooth-On polysulfide rubber, Surgident Neo-Plex Rubber, Permamold Latex, Hysol Epoxy, plaster of Paris, and Coecal plaster. Many similar products could be substituted for those recommended here. However, I have used these extensively and know that they work well.
CASTING TECHNIQUES IN MARINE ARTIFACT CONSERVATION
The first published account of casting in marine conservation as a means of retrieving completely oxidized artifacts is that reported by Katsev and van Doorninck (1966:133-141). Utilizing a lapidary saw, they sectioned small encrustations containing natural molds left by oxidized Byzantine iron tools. Some specimens required only one cut, other more complicated objects required several cuts. The corrosion residue was removed from the natural molds and a piece of cardboard or plastic made to fit between the sawn halves to compensate for the material removed by the saw blade. The mold then was filled with a flexible compound and the halves fitted together. The rubber cast was removed once the compound had cured. When the rubber flashing that formed along the seams of the mold was cut away, a replica of the disintegrated artifact was obtained. These rubber casts are not permanent nor long lasting, but they will last for a number of years. Their life and usefulness can be extended by storing them in plaster mother molds to provide support and keep them from stretching and losing their form. If a permanent epoxy cast is needed, then a mold has to be made of the polysulfide rubber cast, and then this mold cast in epoxy.
After casting several molds sectioned with a lapidary saw, we noticed several disadvantages. The technique is limited to small encrustations and to uncomplicated shapes which require only a few cuts. A problem also arises in correctly aligning the two halves and the cardboard gasket required to replace the thickness sawn away by the saw blade. This problem is compounded when more than one cut is made. When the mold is cut with a saw, the seam flashing is very noticeable. If X-ray facilities are available, some of the problems of casting natural molds can be overcome. The radiographs reveal the shape of the object and extent of the corrosion. In certain encrustations it is possible to use a pneumatic air chisel to cut openings into distal ends or key points of an object. Through these holes, the corrosion residue can be washed out and the rubber compound poured. Alternatively, the air chisel can be used to inscribe a line along or around an encrustation. By hitting along this line with a chisel and a hammer, the encrustation can be broken in a predetermined manner. Simple encrustations are easily opened and cast this way. I do not recommend using a lapidary saw to open natural molds in encrustation, it is much more effective to break them open and cast the void with epoxy.
On most marine sites, the only way to recover a number of the smaller, thin, iron artifacts is to cast the natural mold left inside the encrustation after they have corroded to a slush. The corrosion residue can be remove, sometimes as simply as washing it out with water, and other time it take a considerable amount of picking. After it is removed, the void is filled with epoxy, which eliminates the problems presented with the polysulfide rubber molds. After setting, the encrustation can be removed with a pneumatic chisel, revealing a perfect replica of the original iron objects. By this technique we have been able to cast in epoxy the corroded hammer heads directly onto the original wooden handle, as well as iron cleaver hafted onto the original wooden handle, a variety of iron keys, and several door locks (Figure 11). See Hamilton (1976:72-85) , North (1987:231-232), and Muncher (1988) for a more complete discussion of the techniques of casting and it value when applied to the material from marine sites. It can be emphatically stated, that is casting techniques are not being utilized, a significant amount of data is going to be lost. Natural molds of disintegrated metal objects are often encountered in a very large encrustation, where they cannot be detected on radiographs even if they could be X-rayed. To avoid destroying possible valuable information, close observation is required when the encrustations are being taken apart with the air chisels to detect the molds before they are destroyed. Because of the presences of these natural molds in large encrusted metal objects, the use of acids or even electrolysis to remove encrustation (Montlucon 1986, 1987) is not recommended for general use. When molds are found, it is possible to open a small area on one side, clean it out, and fill it with epoxy. This opportunity is lost, if "deganguing" were used without some discretion. The casting examples discussed above involved iron artifacts, however, similar casting procedure are often employed on silver artifacts which often corrodes extensively in anaerobic marine environments. Casting techniques also have been extremely useful in recovering stamps from corroded silver specimens. From two 16th-century Spanish shipwrecks there were a number of silver discs which are usually sand cast, circular, and plano-convex in cross section. On the flat surface of the silver discs are usually one or more stamps indicating ownership, mines, and tax marks. Frequently the stamps are obliterated in the corrosion process. The encrustation, however, forms a perfect mold of the original surface of the silver and a reverse impression of the stamps remains in the encrustation. In one example the encrustation on a singularly encrusted silver disc was removed with an air scribe by chipping along the circumference and separating the two halves of the encrustation from the silver. The reversed silver stamps were revealed by carefully removing the corrosion products from the interior surface of the encrustation with fine bristle brushes and pointed wooden sticks. A latex peel was made of the interior surface of the encrustation which contained the reverse of the stamp. Plaster casts were made from the latex peel of the stamp impression and the stamps were highlighted with a soft-lead graphite pencil. It is possible to recovered many otherwise lost stamps through this procedure and it is routinely incorporated into our conservation of encrusted silver discs in order to preserve this valuable data. Few historians or archaeologists would deny that the salvage of the stamps is historically more significant than the silver with its stamps obliterated.
Out of many casting problems encountered the examples discussed above unquestionably present a strong case for the value and significance of casting in the conservation of marine shipwreck material. The recovered data are of the type that is lost daily by improper care and conservation of archeological material. They emphasize the reasons why marine shipwreck material should be processed by personnel
familiar with the material culture and the alternative techniques of salvaging and preserving the maximum amount of data. There are many ways of utilizing casting techniques during the conservation of shipwreck material. The important thing is that some knowledge of the procedures be had and the necessary supplies and casting compounds be kept ready for use.
CONCLUDING REMARKS
This paper has attempted to present the current state of conservation of archaeological material from marine environments. Various requirements, equipment, chemicals, and procedures have been discussed, but many more were not. There are many minor variations, optional steps, and tricks that are used and learned by each practitioner. Time and space did not allow a thorough discussion of each technique and the variations within the different techniques; therefore, there is no way around consulting the original, more exhaustive published sources. Individuals interested in archeological conservation should consult the referenced sources and a trained conservator before attempting the procedures described herein. The preservation of antiquities should produce objects that are chemically stable with an aesthetically acceptable appearance. All treatments should be reversible in the event that the object should require additional preservation. Just because an object has been successfully conserved, does not mean it will not deteriorate in the future. Only if stored or displayed under optimum conditions can stability be assured. Metal artifacts, as well as those made of organic or siliceous material can become chemically unstable from a myriad of causes and require periodic inspection and evaluation, as well as possible retreatment. At our present stage of knowledge, perhaps it is most realistic to say that the objective of archeological conservation is to delay reprocessing as long as possible by proper storage and to make any necessary retreatment simple and brief. It is obvious that the conservation laboratory can play a major role in archaeology, if the objective is to produce the maximum amount of archeological data from the excavation of waterlogged and underwater sites. Conserving the recovered artifacts is just one of the steps. During the course of this discussion a clear idea of the facilities required, the treatments available, chemicals utilized, and various insights on conservation have been presented which should be helpful in evaluating any conservation proposal or for assistance in establishing conservation facilities designed to conserve the vast array of material found on marine shipwreck sites. Estimating the costs involved is more complicated. Still with a knowledge of what is needed, it is just a matter of determining for each facility, the equipment required to start off with, the level or volume expected of the laboratory, the variety of treatments to be done and the figures fall into place. All the treatments discussed are used to conserve material from marine sites. With most, it is not a question or matter of which is preferred or which is better than another. The fact is that a one of every treatment discussed here would be the preferred means of treatment of a given artifact. For that reason, a conservation laboratory has to have a conservator familiar with the various treatments, know on what occasions and situations they are the most appropriated, and have the facilities, equipment, and chemicals to carry out the treatments.




